How does Covaxin compare with Covishield, Which is better ?

Indian Government started the vaccination rollout on 16th January 2021 and so far around 1. 8 crore people (as of 4th March) have been vaccinated with priority to healthcare and other frontline workers. From 1st March, the drive at the private vaccination centers has begun with the Prime Minister also taking the vaccine. As we are all aware by now, 2 vaccines produced in India i.e. “Covishield” and “Covaxin” have been authorized for emergency use in India. In near future when choices may be given to the recipients, the most important question will haunt people; “which vaccine to prefer?” On 3rd March, Covaxin phase-3 interim results were also declared and therefore head-to-head comparison is now possible. Firstly, it is important to understand basic differences between Covishield and Covaxin in a comprehensive manner and then make interpretations.
Covishield vs Covaxin comparison:
1. Biological Components:
Covishield is a viral vector vaccine. It uses a weakened, non-replicating strain of Chimpanzee cold virus (adenovirus) to carry genetic material of the spike protein of SARS-CoV-2 into human cells. Covaxin contains an inactivated SARS-CoV-2 (Strain: NIV-2020-770) which is disabled for replication. However, the proteins are intact which are able to provoke immunity of the host. There are other chemical ingredients in the form of excipients which are detailed out in the table below. If you want to understand these vaccine types further, read another article on Covipedia by clicking the link below:
Overview of types of vaccine
2. Chemical Ingredients:

3. Mechanism of Immunization
Covishield – This vaccine produces antibodies against only a specific region of the virus. It contains a portion of the DNA that codes for the spike protein (S-protein). Once inside the cells, the DNA part first needs to enter the nucleus to create its mirror image (complementary RNA). Then this RNA comes out in the cytoplasm as a messenger and starts making S-protein through a machine available for this purpose called ribosome. Since it is S-protein that provokes immunity it may not be as close to natural immunity as created by Covaxin. If there are any long-term side effects of the DNA material remaining inside the nucleus (e.g. integration in human DNA) is not yet known. So far, DNA vaccines were only being tried out for treating cancer patients and never used for preventing infections in normal subjects.
Covaxin – This vaccine can produce antibodies against many regions of the complete virus. Since this vaccine contains a full inactivated virus with all its 29 proteins intact, the immunity provoked by it will be more comprehensive and closer to natural immunity arising out of an infection. This does not contain any genetic material that can either replicate or go inside the nucleus but provokes the immunity against virulent proteins other than S-protein as well. This uses a tried and tested technology platform used by other vaccines like polio vaccine. However, these vaccines require an adjuvant to provoke immunity. For this purpose, alum is commonly used which mainly provokes Th-2 type immunity which also leads to more side effects. Hence, Bharat Biotech has used an alternative adjuvant “Algel-IMDG (Imidazoquinolinone)” which stimulates Th-1 type immunity that is also generated by mRNA/DNA vaccines.
4. Clinical Development: Covishield has been developed by AstraZeneca with Oxford university in the UK and is being manufactured by the Serum Institute India (SII) in Pune. Covishield has completed phase 3 trials in S. Africa, Brazil and UK. 90% of the subjects in these studies were under the age of 55 making the efficacy and safety data applicable to this age group. The company has presented bridging study results in Indian population to the regulatory authorities based on which the approval was granted by DCGI. This data is not yet available in the public domain. Covaxin has been developed by Hyderabad based Bharat Biotech along with the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV) in Pune. The phase 1 and phase 2 studies enrolled 375 people and phase 3 study enrolled 25,800 participants between 18-98 years of age, including 2,433 over the age of 60 and 4,500 with comorbidities making it the largest clinical trial in India.
5. Dosage Regimen: Covishield has been recommended to be taken in 2 doses. Observation of data from the UK shows improved protection with a gap of 12 weeks between 2 doses; though currently the 5 March 2021 For internal circulation Page 3 of 6 expert committee set up by the Drug Controller General of India (DCGI) has recommended a gap of 4 weeks. Covaxin has been recommended to be taken in 2 doses 4 weeks apart.
6. Efficacy: Covishield has an average efficacy of 70% when 2 doses are administered 4 weeks apart. This data is from a meta-analysis (pooled analysis of multiple studies) of 4 Covishield trials in 11,636 patients out of which 3 trials were single blind and one double blind in 3 different countries. The efficacy of Covishield was published in The Lancet (link to the article). Observation of data has shown that the efficacy improves as the gap between the 2 doses is increased reaching a reported efficacy of 82.4% with a 12-week gap. Since, the phase-3 trials were conducted with a 4-week interval, it has become the standard. Covaxin phase-3 interim results show an efficacy of 81%. This data is from one double blind study of 28,500 patients in India. Thus, from efficacy angle, Covaxin scores higher than Covishield with more robust and coherent data in Indian subjects.
7. Protection against Mutations: Preliminary research shows both vaccines are effective against the variant of the novel coronavirus first detected in the UK but there is no data on their efficacy against the mutants found in South Africa and Brazil. Data against these 2 variants is yet to be generated for both these vaccines.
8. Side Effects:
Based on the fact-sheets released by both manufacturers:

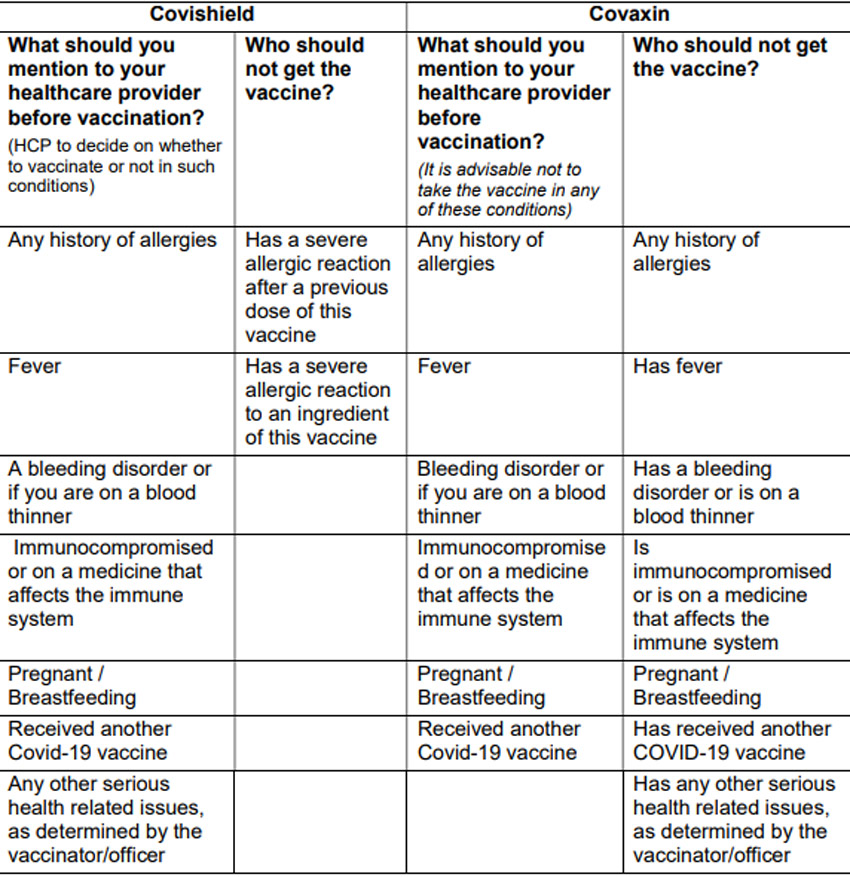
9. Precaution and Contraindications:
Based on the fact-sheets released by both manufacturers:
10. Consent: Covishield does not require any consent form as it has completed the phase-3 clinical trials. 5 March 2021 For internal circulation Page 5 of 6 Covaxin – Since it was approved by the Indian regulatory authorities before phase 3 results were available, it is being administered to people in a large clinical trial setting. This is being termed as “clinical trial mode”. Such use is similar to some of the anti-cancer drugs which have been used on compassionate basis before it’s formal approval. This requires pre-informed consent of the patient who is explained that it is not yet approved and he provides his consent for the same. This form states that the beneficiaries will be provided care in government authorized hospitals if they faced side-effects from the vaccine. They will also reportedly get compensated if they face adverse effects from the vaccine. Now that the phase 3 efficacy data is available, once it is presented to the authorities and restricted emergency use is granted the need for informed consent will be removed.
11. Price:
While the vaccine is being given for free at the government institutions, the price at private institutions has been capped at ₹250 per dose. Currently, the person does not get to choose which vaccine he/she would receive. It is likely that once the vaccines are available for private market sale, choice of brand will be given to the people.
Recommendation: At the face value, the stellar reputation of Oxford University, AstraZeneca and Serum Institute of India is daunting enough to prefer Covishield if a choice is given to you. However, an objective comparison of properties, attributes and available information indicates that Covaxin is better placed to receive our recommendation for the following reasons: Higher efficacy of Covaxin comes out of robust data from one double blind controlled clinical trial as compared to lower efficacy of Covishield with results pooled from multiple studies that were not blinded and were dissimilar. Covaxin also seems to have distinct safety advantage with the lack of anaphylaxis and potential for neurological adverse events with Covishield. Two cases of transverse myelitis in the UK had stalled the clinical trial for some time but resumed once the safety was reconfirmed.
On the regulatory front, Covishield (its original codename of AZD1222) seems to have an advantage over Covaxin being approved in European countries and Australia whereas Covaxin has been approved in India and Zimbabwe so far. Also, by now millions of doses have been used in India and Europe without any major concern. Covishield may therefore be a natural choice for those who value external endorsement (in this case UK, EU, Australia). However, without extraneous considerations on the strength of head-to-head comparison shown above Covaxin is a compelling choice.
Authored by Dr. Pranit Desai, Dr. Kshipra M. Gharpure, reviewed by Dr. Chitra Bargaje and edited by Dr. Dhananjay Bakhle (Medical Research)
See What’s Next in Tech With the Fast Forward Newsletter
Tweets From @varindiamag
Nothing to see here - yet
When they Tweet, their Tweets will show up here.






























